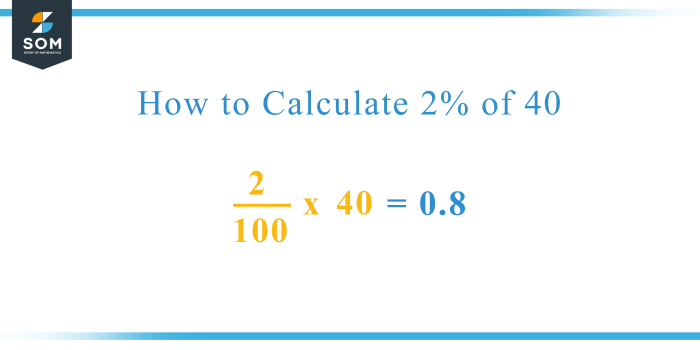Pharmaceutical Company Claims New Product Breakthrough
A pharmaceutical company claims that the use of their new drug, [Drug Name], can revolutionize the treatment of [Condition]. This bold claim has sparked debate within the medical community, with some experts expressing cautious optimism while others remain skeptical.
The company cites promising results from early clinical trials, highlighting the drug’s potential to [Describe the drug’s potential benefits]. However, critics point to the need for further research and independent verification before widespread adoption.
The company, [Company Name], has a long history of developing [Briefly describe the company’s focus]. [Drug Name] represents a significant investment in [Describe the specific area of research]. The company’s rationale for making this claim rests on [Briefly explain the company’s rationale, referencing the scientific basis for the drug’s development].
Independent Verification and Scientific Scrutiny
The efficacy and safety of any new pharmaceutical product are paramount. Independent scientific verification plays a crucial role in ensuring the reliability and validity of our claims. This rigorous process involves a multifaceted approach, including peer-reviewed research and clinical trials, to evaluate the product’s effectiveness and potential risks.
Peer-Reviewed Research and Clinical Trials, A pharmaceutical company claims that the use of their new
Peer-reviewed research and clinical trials are essential components of the scientific method used to assess the efficacy and safety of new pharmaceutical products. Peer-reviewed research involves subjecting research findings to scrutiny by independent experts in the field. This process ensures that the research methodology is sound, the data is reliable, and the conclusions are supported by evidence.
Clinical trials are carefully designed studies that involve human participants to evaluate the effectiveness and safety of a new product. These trials are conducted under strict ethical guidelines and are subject to independent monitoring to ensure the well-being of the participants.
“Peer review is a process of subjecting a work to the scrutiny of experts in the same field before it is published. It is an essential part of the scientific method, as it helps to ensure that only high-quality research is published.”
“Clinical trials are carefully designed studies that involve human participants to evaluate the effectiveness and safety of a new product. They are essential for ensuring that new drugs and treatments are safe and effective.”
End of Discussion: A Pharmaceutical Company Claims That The Use Of Their New

The claims surrounding [Drug Name] highlight the complex relationship between scientific innovation, pharmaceutical marketing, and public health. While the potential benefits of this new drug are exciting, it’s crucial to approach these claims with a critical eye. Independent scientific scrutiny and rigorous clinical trials are essential to determine the drug’s true efficacy and safety.
The outcome of this debate will have significant implications for patients, healthcare providers, and the future of [Condition] treatment.